Image: To produce electricity, the fuel cell works by exploiting the energy of an electrochemical reaction between a fuel, such as hydrogen, and an oxidant, such as oxygen. Unlike batteries, to operate continuously, fuel cells require a supply of fuel and oxidant in real time.
The story begins at the end of the 18th century, when British scientist William Nicholson (1753-1815) discovered that water could be broken down into hydrogen and oxygen by electricity. But it was at the beginning of the 19th century that the foundations of the modern fuel cell were laid.
In 1839, British physicist William Grove (1811-1896) invented the first working fuel cell, known as the Grove cell. It used hydrogen as fuel and oxygen as oxidant, and produced water and electricity as reaction products.
Over the following decades, several chemists made significant contributions to fuel cell technology, Francis Thomas Bacon (1904-1992), Wilhelm Ostwald (1853-1932), Karl Kordesch (1922-2011).
In 1889, German researcher Ludwig Mond (1839-1909) developed a gas-based fuel cell, using carbon monoxide as fuel.
In 1965, the Gemini 5 space mission was the first to use a proton exchange membrane fuel cell (PEMFC) aboard its spacecraft.
Since then, different types of fuel cells have been developed, including alkaline, PEMFC, solid oxide and molten carbonate fuel cells, each of which has advantages in various fields, such as space, electric vehicles, back-up systems, etc. emergency and stationary applications.
The use of the term "fuel cell" emphasizes the electrochemical process that occurs inside the device, rather than the specific type of fuel used. The fuel cell provides electricity continuously as long as it is supplied in real time with fuel and oxidant. Unlike batteries, the fuel cell does not store electrical energy and therefore does not need to be recharged. On the other hand, it is necessary to store and transport the fuel appropriately for continuous use.
The term "fuel" is used broadly to encompass different types of fuels that can be used, such as hydrogen (H2), methanol (CH3OH), methane (CH4), propane (C3H8), gas synthetic, a mixture of carbon monoxide (CO) and hydrogen (H2), etc. The general terminology of "fuel cell" allows other chemical compounds to be included in the future.
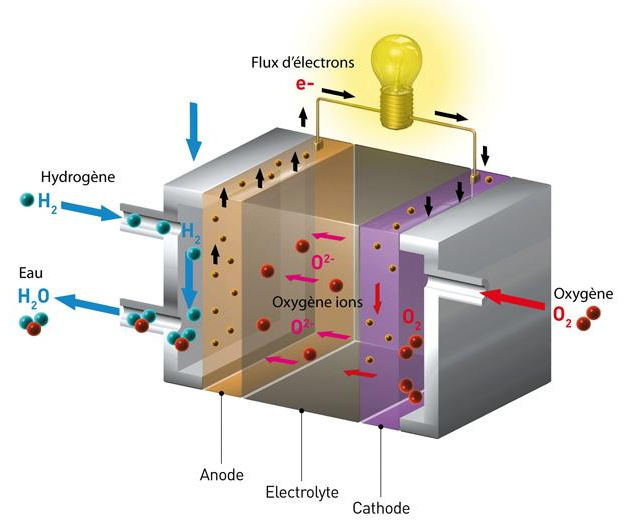
The hydrogen-oxygen fuel cell, also known as a proton exchange membrane fuel cell (PEMFC), requires a continuous supply of hydrogen and oxygen.
Hydrogen is supplied to the anode (negative electrode), while oxygen (often air) is supplied to the cathode (positive electrode).
The two electrodes are separated by an ion-conducting polymer membrane, called the electrolyte.
At the anode, the hydrogen decomposes into hydrogen ions (protons H+) and electrons (e-) through a dissociation reaction, catalyzed by a material such as platinum. The catalyst at the anode facilitates this reaction (2H2 → 4H+ + 4e-).
The protons are then transported through the proton exchange membrane (PEM) to the cathode, while the electrons are forced to flow through an external circuit, creating an electric current that can be used to power electrical devices.
Thus, the electrons provide electricity, then join the cathode where they take part in the reduction reaction with the protons and the oxygen to form water.
The catalyst at the cathode, usually platinum alloyed with other metals, facilitates this reaction (O2 + 4H+ + 4e- → 2H2O).
The water produced at the cathode, as well as the excess hydrogen and unconsumed oxygen, are evacuated from the fuel cell in the form of water vapor and air. The general equation for the chemical reaction in a hydrogen-oxygen fuel cell is 2H2 (hydrogen) + O2 (oxygen) → 2H2O (water).
Hydrogen is the most abundant element in the universe, but it is not available as free hydrogen on Earth. However, it can be produced from various sources (electrolysis of water, reforming of hydrocarbons, etc).
Hydrogen-oxygen fuel cells have high energy efficiency, which means that they can convert much of the chemical energy contained in hydrogen bonds into electricity.
These fuel cells can convert between 40% and 60% of the chemical energy of hydrogen into usable electricity. The rest of the energy is dissipated as heat. For comparison, the efficiency of traditional internal combustion engines is between 25% and 40%.
In water, the binding energy is relatively high compared to other molecular binding energies. The interaction between hydrogen atoms and oxygen atom forms a polarized covalent bond. This polarity strengthens the interaction between the atoms, thus increasing the binding energy. In the water molecule, oxygen is more electronegative than hydrogen, which means that it attracts the shared electrons more strongly into the bond, thus creating a negative partial charge on the oxygen and a positive partial charge on the hydrogens. The average covalent bond energy between the hydrogen atom and the oxygen atom is about 460 kilojoules per mole (kJ/mol). Part of this energy is converted into electrical energy through the process of electrochemical reaction in the fuel cell.
Thus, hydrogen is considered the ideal fuel for fuel cells due to its high potential for energy efficiency and emission reduction.