The lights of the Sun | ||||||||||||||||||||||||||||||||||
Rainbow of the wavelengths |  Automatic translation Automatic translation | Updated September 17, 2014 | ||||||||||||||||||||||||||||||||
The Sun emits a plurality of electromagnetic waves, to far ultraviolet (FUV) such as gamma rays (higher frequencies) to radio waves (lower frequencies), through X-rays, ultraviolet rays, visible light, infrared radiation, microwaves. These electromagnetic waves, whose vector is the photon, move at speed of ≈300000 km/s. With our eyes, we see only the wavelengths in the visible range between 400 and 800 nm, but when it comes to shorter or longer wavelengths, we need to use specialized devices. Specialized instruments are usually ground or space telescopes equipped observing light in different wavelengths. | The telescopes make use of this valuable information wavelength by embarking instruments such as spectrometers who observe several wavelengths simultaneously and measure the quantity of present elements to each wavelength. | 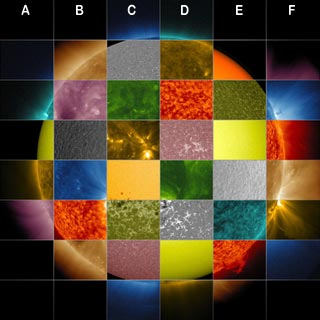 Image: this composition exhibits various aspects of the surface or the atmosphere of the Sun into 10 different wavelengths, invisible to the naked eye. These special lights sun is converted and colorful by the telescope SDO (Solar Dynamics Observatory) so that humans can see them. The observed object here the Sun appears in a beautiful "rainbow sky" colors representing the light of the Sun. More the temperature is higher, more the dominant color moves towards microwaves to gamma waves. The yellow light has a wavelength of ≈580 nm, it is typically from about atoms, heated to about 5700 ° C, which is the case of the surface of the Sun. Extreme deep ultraviolet lights such as gamma waves have a wavelength of ≈9 nm, it is usually colorized green in the SDO images, it usually comes from the atoms heated to about 6,300,000 ° C which is the case of solar eruptions which can reach temperatures as high. Credit: NASA / SDO / Goddard Space Flight Center | ||||||||||||||||||||||||||||||||
The wavelengths of the Sun | ||||||||||||||||||||||||||||||||||
The telescopes can collect light in ranges of frequencies inaccessible to us. | Video: Sun characteristics appear radically different when viewed in different wavelengths. |
NB: Between the wavelength (λ) and frequency (ν) is the following relationship: ν = c / λ ν = wave frequency in hertz c = speed of light in vacuum in m / s λ = wavelength in meter | ||||||||||||||||||||||||||||||||
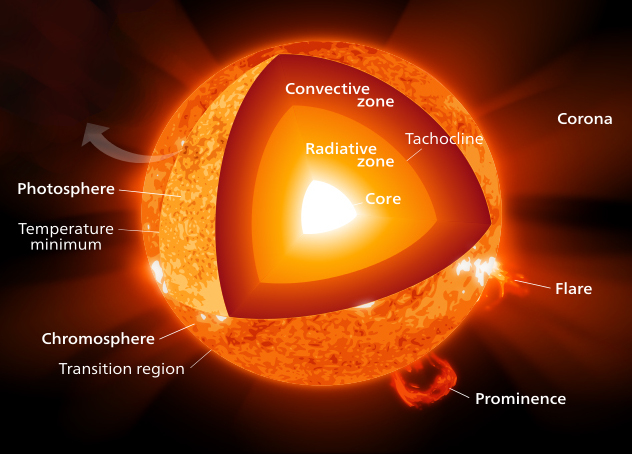
The different layers of the Sun | ||||||||||||||||||||||||||||||||||
Core of the Sun is the zone where the nuclear reactions occurs (fusion of hydrogen atoms). At the center of the Sun the temperature reaches about 15 million degrees and pressure 22,100 billion Pascals (Pa). By comparison the pressure of Earth's atmosphere varies around 100,000 Pa. | Photosphere 160 km thick is only responsible for the emission of energy that bathes the planet, it spotted granules. | 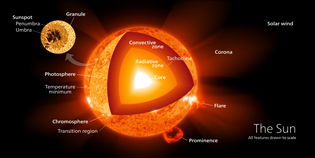 | ||||||||||||||||||||||||||||||||
"The data available on this site may be used provided that the source is duly acknowledged."



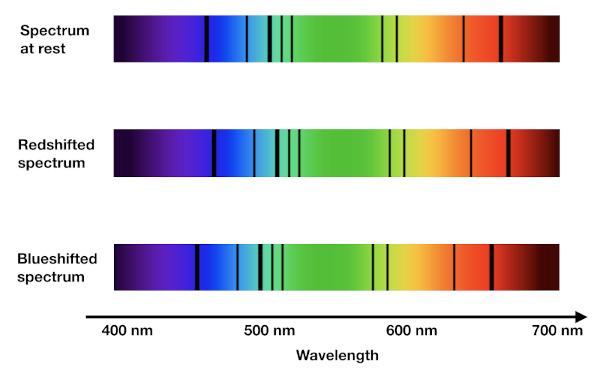 Redshift calculation (z)
Redshift calculation (z)
 Spectacular airglow in France
Spectacular airglow in France
 Light, all the light of the spectrum
Light, all the light of the spectrum
 The spicules of the Blue Sun
The spicules of the Blue Sun
 Global dimming
Global dimming
 Solar pillar, a link between sky and earth
Solar pillar, a link between sky and earth
 The speed of light and space-time
The speed of light and space-time
 The Universe of X-rays
The Universe of X-rays
 Diamond rings above the Pacific
Diamond rings above the Pacific
 The incredible precision of the second
The incredible precision of the second
 Effects of light aberration
Effects of light aberration
 Radioactivity, natural and artificial
Radioactivity, natural and artificial
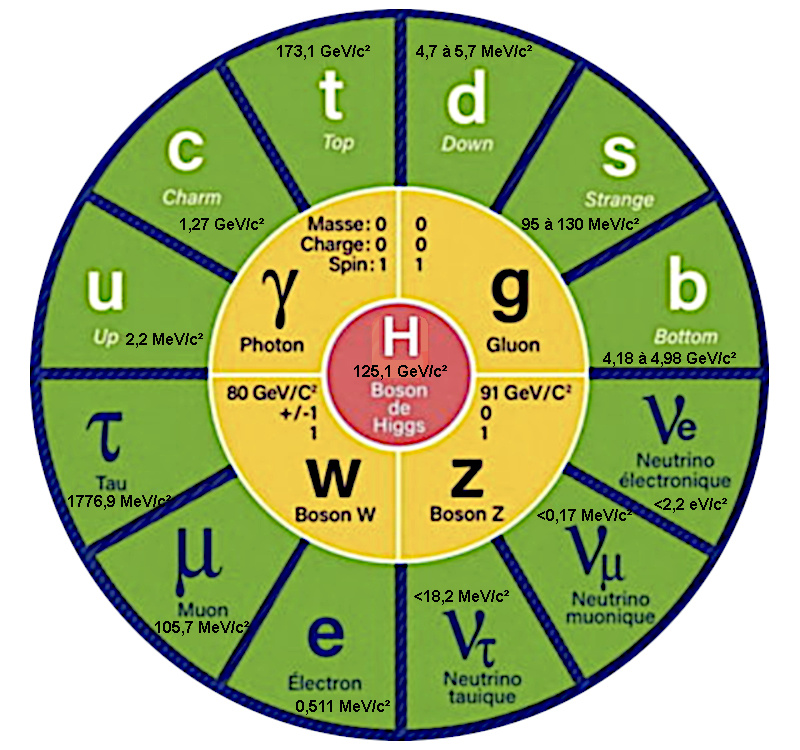 Why do elementary particles have no mass?
Why do elementary particles have no mass?
 The shadow of the black hole
The shadow of the black hole
 Dawn and its rays of light
Dawn and its rays of light
 The Blue Moon
The Blue Moon
 Gravitational illusion or gravitational lens
Gravitational illusion or gravitational lens
 The incredible illusion of the same color
The incredible illusion of the same color
 Perfect storm and devastating effects
Perfect storm and devastating effects
 The infernal journey of the photon
The infernal journey of the photon
 The power of the Sun
The power of the Sun
 Bioluminescence of living organisms
Bioluminescence of living organisms
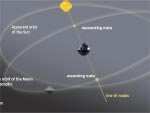 Eclipses explained by the plane of the orbit
Eclipses explained by the plane of the orbit
 Super Moon
Super Moon
 Laser light
Laser light
 We do not see with our eyes but with our brain
We do not see with our eyes but with our brain
 Differences between heat and temperature
Differences between heat and temperature
 Big Moon Illusion
Big Moon Illusion
 Zodiacal light, the diffuse white glow
Zodiacal light, the diffuse white glow
 Explanation of the 8 of the analemma
Explanation of the 8 of the analemma
 The colors of the rainbow
The colors of the rainbow
 Shadow of the Earth anti-twilight ark
Shadow of the Earth anti-twilight ark
 How many photons to heat a cup of coffee?
How many photons to heat a cup of coffee?
 Spectroscopy, an inexhaustible source of information
Spectroscopy, an inexhaustible source of information
 The Cherenkov light
The Cherenkov light
 The lights of the Sun
The lights of the Sun
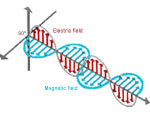 What is a wave?
What is a wave?
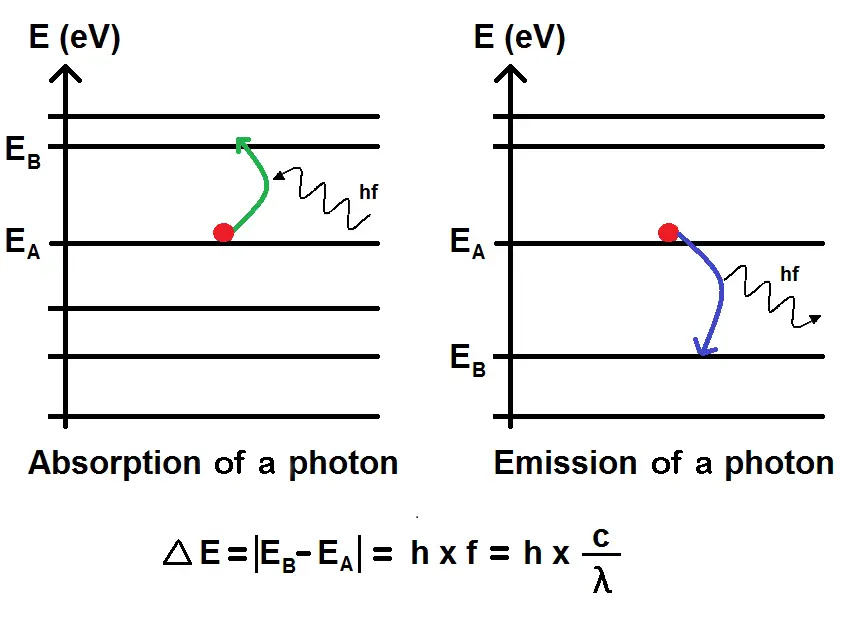 Planck's equation and black body light
Planck's equation and black body light
 Energy Conservation
Energy Conservation