Nuclear reactions (transformations involving atomic nuclei) represent the most energetic processes in the universe. Two main pathways exist to release this energy contained in matter: fission and fusion. Although radically different in principle, both reactions obey Albert Einstein's (1879-1955) famous equation: \(E = mc^2\), which establishes the equivalence between mass and energy.
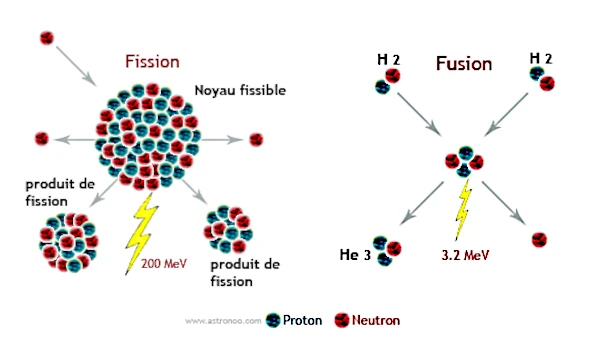
Fission involves the splitting of a heavy nucleus (such as uranium-235 or plutonium-239) into two lighter fragments upon neutron impact. The released energy comes from the mass difference related to the binding energies per nucleon. Typically, the fission of \(^{235}\)U releases about \(200\ \text{MeV}\) per individual nuclear fission reaction, after capturing a neutron.
Discovered in 1938 by Otto Hahn (1879-1968) and Fritz Strassmann (1902-1980), and interpreted by Lise Meitner (1878-1968) and Otto Frisch (1904-1979), this reaction releases considerable energy and several neutrons, which can in turn cause new fissions, thus creating a chain reaction.
The released energy comes from the mass defect: the sum of the masses of the fission products is less than the mass of the initial nucleus. This mass defect, although tiny, converts into colossal energy according to \(E = \Delta m c^2\), where \(\Delta m\) is the mass difference and \(c\) is the speed of light.
In summary, the nucleus splits because, beyond a certain size, it is energetically more favorable to exist as two medium nuclei rather than as a single heavy, unstable nucleus. Fission is the expression of this quest for stability, triggered by the addition of a neutron.
Conversely, nuclear fusion involves the union of two light atomic nuclei, such as hydrogen isotopes (deuterium \(^{2}\)H and tritium \(^{3}\)H), to form a heavier nucleus (helium \(^{4}\)He). This process, which powers stars like our Sun, releases even more energy per nucleon than fission. To overcome the electrostatic repulsion between positively charged nuclei (Coulomb barrier), extreme conditions of temperature (on the order of millions of degrees) and pressure are required. The energy released is about \(17.6\ \text{MeV}\) per D-T reaction.
Mastering fusion on Earth represents a monumental technological challenge, but its potential is immense: abundant fuel, low production of long-lived radioactive waste, and no risk of runaway reaction.
Note: When it is said that D-T fusion releases 17.6 MeV, this refers to the total energy per elementary reaction, i.e., for the interaction between a deuterium nucleus (\(^{2}\)H) and a tritium nucleus (\(^{3}\)H). This energy is distributed between a helium-4 nucleus (≈ 3.5 MeV) and a neutron (≈ 14.1 MeV). Since the reaction involves 5 baryons (2+3), the energy per nucleon is: \[ \frac{17.6}{5} \approx 3.5\ \text{MeV per baryon}. \] This value is often compared to other nuclear processes: fission releases ≈ 0.9 MeV/baryon, while fusion reaches several MeV/baryon, hence its superior energy potential on the scale of reactive mass.
The following table summarizes the main characteristics of these two nuclear reactions, highlighting their fundamental differences.
| Characteristic | Fission | Fusion | Comment |
|---|---|---|---|
| Reactants | Heavy nuclei (U-235, Pu-239) | Light nuclei (D, T, He-3) | Limited availability for enriched uranium, abundance of deuterium in seawater |
| Energy released per reaction | ≈ 200 MeV | ≈ 17.6 MeV | Total energy per elementary reaction |
| Specific energy (per nucleon) | ≈ 0.85 MeV/baryon | ≈ 3.5 MeV/baryon | Allows direct comparison of energy efficiency |
| Ignition conditions | Critical mass | Density × Temperature × Confinement time (Lawson criterion) | Fusion requires temperatures on the order of 10^8 K and prolonged confinement |
| Operating temperature | ≈ 300–600°C for thermal neutron reactors | ≈ 100 million K for D-T plasma | Fusion requires extremely hot plasmas |
| Energy efficiency | ≈ 33–37% in current power plants | ≈ 30–50% projected for ITER and DEMO | Efficiency limited by thermal conversion and losses |
| Neutron production | Fast neutrons emitted (≈ 2–3 per fission) | Very energetic neutrons (14 MeV) for D-T | Neutrons can activate materials and cause transmutations |
| Current applications | Nuclear power plants, A-bombs | Experiments (ITER, NIF), H-bombs | Controlled fusion remains experimental |
| Waste | Long-lived radioactive waste | Low or transient radioactive waste (neutron activation of materials) | Fusion generates fewer problematic long-term wastes |
| Risks | Possible serious accidents, criticality, radiological contamination | Low risk of local explosion, neutron activation | Fusion is intrinsically safer than fission |
| Required technology | Thermal or fast neutron reactors, control rods, moderator | Magnetic confinement (tokamak, stellarator) or inertial (laser) | Confinement technologies still experimental for fusion |
| Fuel availability | Enriched uranium or recycled plutonium | Abundant deuterium, tritium produced by lithium irradiation | Deuterium is almost unlimited, tritium is rare and artificially produced |
| Reaction duration | Continuous and controllable in reactors | Stable plasma for a few seconds to minutes in experiments | Fusion is still limited to short confinement durations |
References: International Atomic Energy Agency (IAEA), ITER.