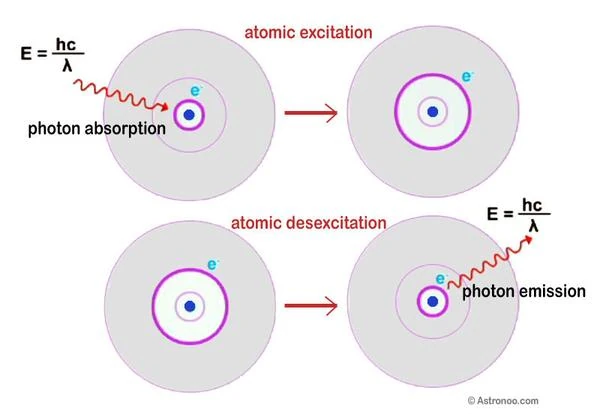
Image description: Principle of absorption and emission of a photon. If the energy involved is moderate, the electronic transitions occur only on the outer layers of the atoms. They correspond to the passage of an electron from the unfilled subshell to an unoccupied subshell of higher energy (absorption) or to the return of an electron to the valence subshell (emission). If the energy involved is sufficiently strong (in very high frequencies) electrons are stripped away. Image source: astronoo.com
Light is the only information scientists have at their disposal to understand the world around us. Over the centuries, scientists have made light "speak."
In 1670, Isaac Newton (1643-1727) looked at the white light of the Sun through a glass prism and noticed that this beam of light was decomposed. He thought that light was composed of corpuscles.
In 1676, Ole Christensen Rømer (1644-1681) determined the speed of light by observing Jupiter's satellites.
In 1690, Christian Huygens (1629-1695) stated that light consists of a series of waves propagated through the ether, an immaterial substrate that serves as a support in the vacuum to convey light.
In 1801, Thomas Young (1773-1829) obtained an interference pattern (image next), showing that light is a wave because waves can add and subtract to create interferences (dark areas interspersed with bright areas). This experiment helps to understand the behavior and nature of light.
In 1814, Joseph Von Fraunhofer (1787-1826) noticed lines in the visible light of the solar spectrum. This German optician and physicist was the first to study the diffraction of light using optical gratings (Fraunhofer diffraction). At that time, the reason for the presence of these lines in the visible spectrum of light was not known. The answer would come much later.
In 1850, Robert Wilhelm Bunsen (1811-1899) and Gustav Robert Kirchhoff (1824-1887) discovered that the spectral lines of light emitted by an incandescent body constitute a signature allowing the identification of this body. By observing the spectrum of solar light, they recognized several chemical elements present on Earth, including cesium and rubidium.
In 1864, James Clerk Maxwell (1831-1879) synthesized electric and electromagnetic waves. He determined that light is an electromagnetic wave and that the entire electromagnetic spectrum is light. What differentiates electromagnetic waves from each other is the wavelength. The different windows of the electromagnetic spectrum are characterized by a range of wavelengths but also by a range of frequencies.
In 1900, Max Planck solved the enigma of the black body; his formula perfectly describes the light emitted by a body as a function of its temperature. In other words, a high temperature indicates high energy, a low temperature indicates low energy.
In 1905, Albert Einstein (1879-1955) explained the photoelectric effect; it is the photons of the incident light that strip electrons from matter.
Photons act as quanta of energy, which Planck had already suggested, but it was Einstein who showed it. These photons therefore have a certain energy, which will strip the electrons from the metal. When we receive the sun's light rays on our skin, we feel the energy they carry.
Light is indeed composed of photons with wave-like behavior, and each of these photons corresponds to an energy. The shorter the wavelength of the photon, the more energetic it is.
In 1911, Ernest Rutherford (1871-1937) specified the structure of the atom and gave a size to the atomic nucleus of the order of 10-14 meters.
In 1913, Niels Bohr (1885-1962) proposed the structure of the hydrogen atom; the electrons are located on quantized orbits. The electron navigates at a certain distance, on one of the onion-skin layers around the nucleus. This is the principle of absorption and emission of light in an atom.

Image description: Young's double-slit experiment is a physics experiment that involves interfering two beams of light from the same source. For this, light is passed through two small slits in an opaque panel. The photons of light pass through the two holes and therefore travel a different path. All those that pass through one hole will arrive together, the waves will add up, so there will be amplification of the light that will gradually mark the photographic screen. Those that pass through the other hole will arrive time-shifted relative to the first and the waves will also add up, but two opposite waves cancel each other out, which is what the black fringes of the interference phenomenon show.
In 1821, Thomas Young (1773-1829), the famous "Young's slits" experiment consists of interfering two beams of light from the same source. This experiment done with photons has been carried out since with all particles. With electrons in the 1920s, with neutrons in the 1950s, with atoms in the 1980s, and with molecules in the 1990s.
N.B.:
Energy of the photon E = hν = hc / λ. E is the energy expressed in joules, h is Planck's constant (6.62 x 10-34), ν is the frequency (number of electromagnetic oscillations), c is the speed of light in a vacuum, and λ is the wavelength. The energy of a photon is therefore infinitely small. In other words, the shorter the wavelength, the higher the frequency and the greater the energy.