Liquid water | ||||
The water is perfect |  Automatic translation Automatic translation | Updated June 01, 2013 | ||
All forms of life based on this alone element, shared by all living things. The water is perfect, it helps to dissolve the materials and chemicals. The water is home to elements that will serve as catalysts for the production of chemicals necessary for life. It is water that we have this fantastic and wonderful diversity of life on Earth. Living organisms are ordered construction of complex molecules that react chemically together. | This weak liaison makes water a very special permanent electric dipole. Hydrogen is produced early in the history of the Universe, it is the first atom to constitute itself, oxygen is an element that appears later, when thermonuclear fusion reactions within stars. Image: If our planet had no relief, liquid water would cover its entire surface to a depth of 3 km. |  | ||
The water molecule | ||||
All atoms are composed of a nucleus (protons and neutrons), carrying a positive electric charge, around which the electrons carry a negative electrical charge. But an atom is globally neutral. | The material Universe is composed of 74% hydrogen, 24% helium, 1% oxygen and other elements combined make up only 1% of the material. Helium is almost chemically inert, it is monatomic in all circumstances, it is the least reactive element and it does not generally form chemical compounds. This explains why the water molecule (H2O), is abundant in the universe. The hydrogen atoms have the option to bind together (H2), or to bind to oxygen atoms (H2O). That is why, apart from the hydrogen molecule, the molecule of water is the most common in space. Image: The water molecule (H2O) is made of two hydrogen atoms (atomic number = 1), connected to an oxygen atom (atomic number = 8), which have their electrons in common. The water molecule has a total of ten electrons which eight were made by the oxygen atom and by two hydrogen atoms. Water is a permanent electric dipole. The electron is not shown here, it has no precise location in the atom as a timeless wave, the electron is both everywhere and nowhere, a little here and a little there. | 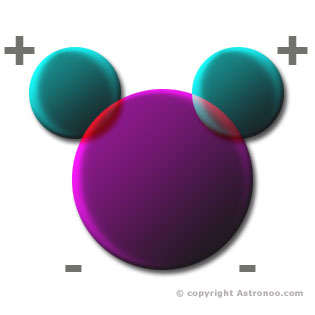 | ||
The states of water and phase transitions | ||||
Pure water exists as a single phase, solid, liquid or gas to a pressure and temperature specific. | 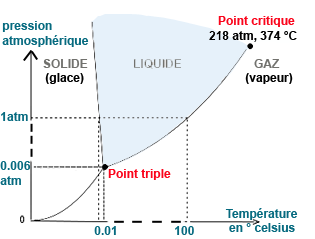 | Image: Diagram of change of state or phase transition of pure water. | ||
"The data available on this site may be used provided that the source is duly acknowledged."



 Artificial intelligence: the explosion of gigantism
Artificial intelligence: the explosion of gigantism
 When AI models train on their own data, they go mad!
When AI models train on their own data, they go mad!
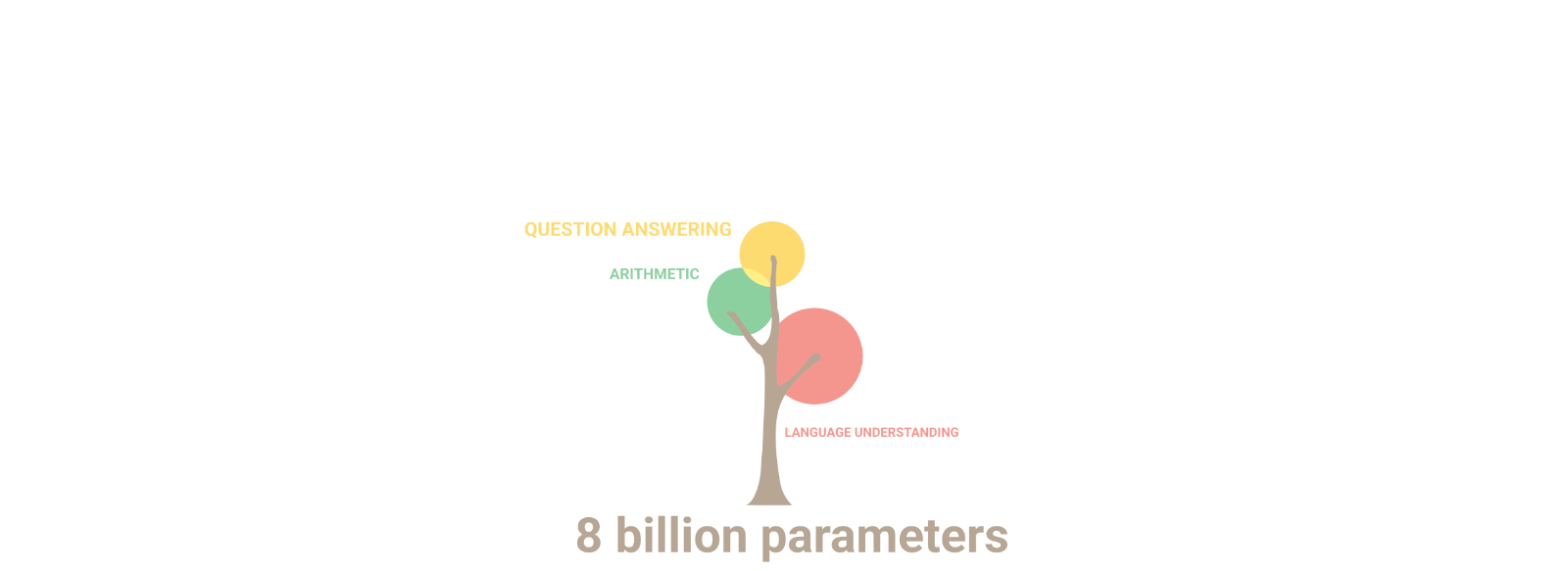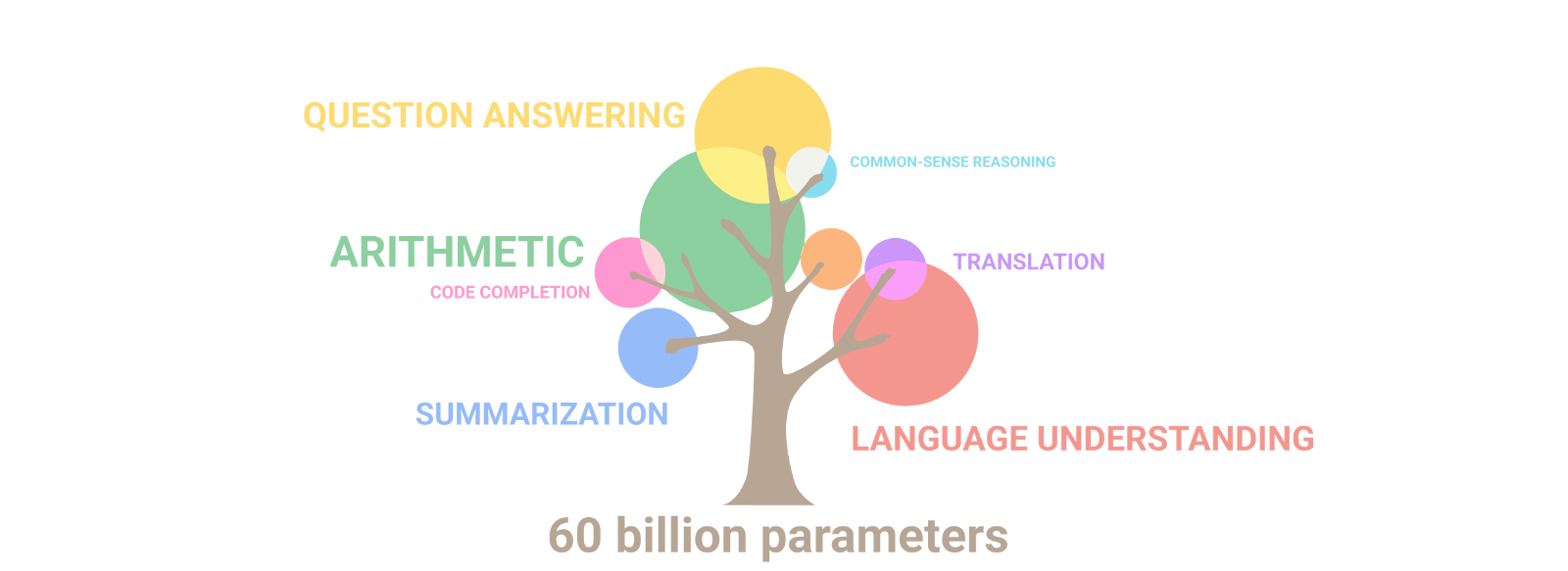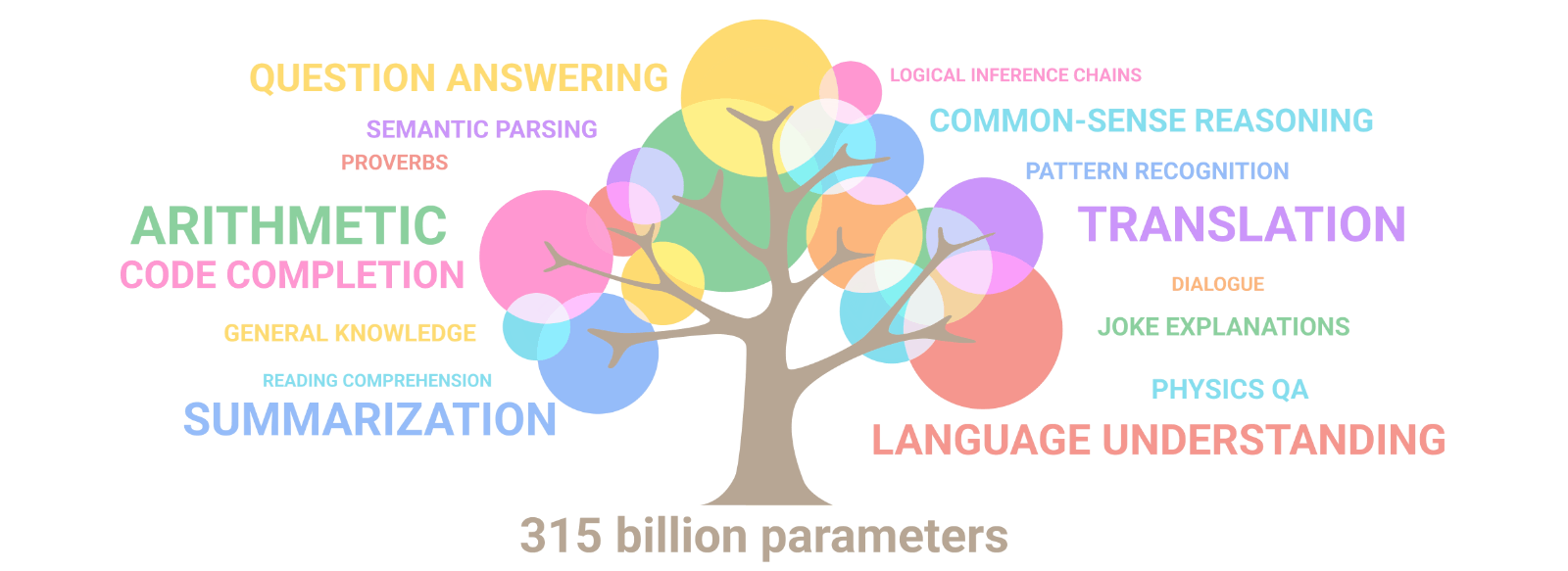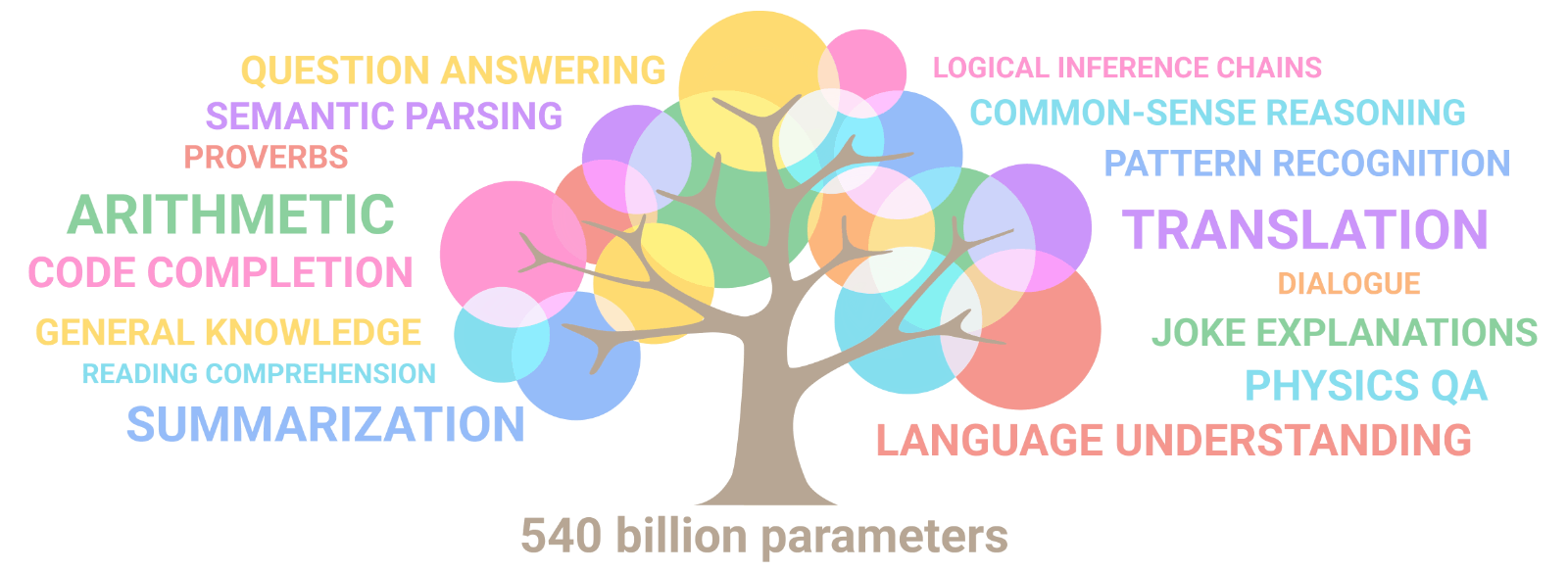 Emergence of artificial intelligence: Illusion of intelligence or intelligence?
Emergence of artificial intelligence: Illusion of intelligence or intelligence?
 The horseshoe crab, a living fossil!
The horseshoe crab, a living fossil!
 Biosignatures or presence of life in the Universe
Biosignatures or presence of life in the Universe
 Challenge and threat of Artificial Intelligence
Challenge and threat of Artificial Intelligence
 How do machines understand, interpret and generate language in a similar way to humans?
How do machines understand, interpret and generate language in a similar way to humans?
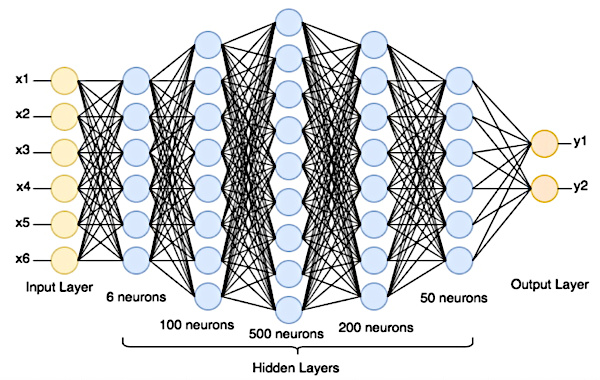 How does an artificial neural network work?
How does an artificial neural network work?
 Origin of life on Earth: Panspermia theory
Origin of life on Earth: Panspermia theory
 Origin of life on Earth: White smoker theory
Origin of life on Earth: White smoker theory
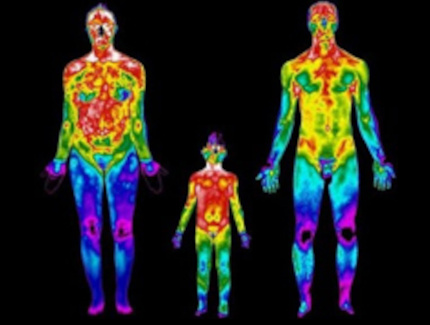 Why 37 degrees Celsius?
Why 37 degrees Celsius?
 Thermodynamics of the sandpile
Thermodynamics of the sandpile
 Are we alone in the universe?
Are we alone in the universe?
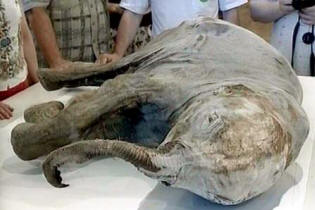 Trace of frozen life in Siberia
Trace of frozen life in Siberia
 Ice cores tell us about our past
Ice cores tell us about our past
 Life evolves in the shelter of glaciations
Life evolves in the shelter of glaciations
 Organ regeneration, the salamander
Organ regeneration, the salamander
 Cosmic rays and the mutation of species
Cosmic rays and the mutation of species
 Mephisto, the little worm of the depths
Mephisto, the little worm of the depths
 Discovery of solid buckyballs in space
Discovery of solid buckyballs in space
 Bipedalism in hominids
Bipedalism in hominids
 Kamchatka giant crab
Kamchatka giant crab
 The passage between the inert and the living
The passage between the inert and the living
 From particles to biochemical life
From particles to biochemical life
 Egocentric vision, the man at the center
Egocentric vision, the man at the center
 Megapod uses volcanic heat
Megapod uses volcanic heat
 Ardi is 4.4 million years old
Ardi is 4.4 million years old
 Natural selection, the birch moth
Natural selection, the birch moth
 The explosion of life in the Ordovician
The explosion of life in the Ordovician
 Liquid water, an accelerator of chemical reactions
Liquid water, an accelerator of chemical reactions
 Neandertal
Neandertal
 Asimo the future humanoid
Asimo the future humanoid
 Conditions for the appearance of life
Conditions for the appearance of life
 Fermi's paradox or Plato's cave
Fermi's paradox or Plato's cave
 The Tardigrade, the immortal animal
The Tardigrade, the immortal animal
 Toumaï, 7 million years old
Toumaï, 7 million years old
 Border between inanimate and living
Border between inanimate and living
 The incredible life of the abyss
The incredible life of the abyss
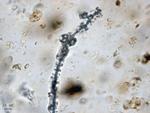 Cyanobacteria create toxic gas
Cyanobacteria create toxic gas
 The short history of the evolution of life
The short history of the evolution of life
 The smallest frog in the world
The smallest frog in the world
 The explanation of the Little Ice Age
The explanation of the Little Ice Age
 Ashen light, the proofs of life
Ashen light, the proofs of life
 Bioluminescence of living organisms
Bioluminescence of living organisms
 Beyond our senses, the great scientific revolutions
Beyond our senses, the great scientific revolutions
 The primitive soup
The primitive soup