We share with the objects of the universe, the same building blocks, the same particles we are stardust. The particles that compose us are assembled in conglomerates of atoms in macromolecules that are the basis of cellular function of living organisms. The structures and forms of these proteins will condition their functions.
Based on our current knowledge, there he has a conceptual definition of life?
Apparently not, yet this is a fundamental question, everyone seemed to know what life is, but nobody really knows answer nor biologists nor doctors nor biochemists nor physicists nor the exobiologists and even less philosophers. Even more surprising, they do not like at all this question because behind the term "life", there is a metaphysical resonance.
Indeed, it is very difficult to define a fundamental concept, like life, time or matter...
Then talk about the living that observed on Earth. What is the current definition of the living or living matter?
Again the experts do not give undeniable conceptual definition but list a series of properties that define the living. Often these definitions contain the term "life" and thus presupposes the idea or the existence of life. In other words these are not definitions of life but life metaphors.
Examples of definition of life or living matter:
- Life is the mode of existence of living bodies.
- Living beings include all organizations which populate our planet and are endowed with life.
- Life is a chemical process in which living organisms are derived.
- A body is said living when exchange of the material and energy with its environment by maintaining its autonomy when it reproduces and evolves by natural selection.
- According to NASA, is alive any system defined spatially by a semi-permeable membrane of his own making and capable of self-sustaining and to reproduce by producing its own components, from energy and / or from external elements.
In these definitions, we understand that life would be a self-organizing system, a mysterious mechanism linked to the material. But this shows that we do not yet know what the exact nature of life and where is the division between the living and the nonliving. | | But what is the mechanism that creates the conditions for life to start and complexity?
A living being, compared to inanimate objects, is a chemical system which itself forms its own substance from the one he draws in the middle.
There is no difference between inert and living matter yet, living matter alone will obtain the energy that it needs to self-replicate, this is where the mystery resides.
The living and the nonliving are made of the same material, one that created the stars, galaxies, nebulae and planets.
On Earth, the transition from inanimate matter to life was done probably in the water, there ≈4 billion years, when the first organic molecules are reproduced.
Quickly on Earth proto-bacterial life appeared less than a billion years after its formation. But a proto-bacteria is already an advanced stage of life because in this simple organism, all functions are proving extremely complex, especially that of replication.
The observation of life on Earth shows us the ability of the material to gradually climb the ladder of complexity. But where is the breaking point between the living and non-living?
In other words what is the assembly of molecules that allows the start of life?
The carbon is produced by nucleosynthesis (fusion of 3 helium nuclei) in the heart of massive stars, then released into space when they explode. Our chemistry, one that constitutes us, started with a small molecular edifice built on a carbon skeleton.
Scientists are trying in vain to create life, based on carbon molecules especially 12C which is a biological signature.
The other voice explored is molecular archeology, with the aim to find the primordial molecules fossilized, but the oldest traces of life (fossilized bacteria) stop there ≈3.5 billion years. In the older sediments, archeobiologists found organic molecules enriched in 12C dating from ≈3.8 billion years. Again life hides us its secrets, so scientists are turning to space and Mars in the hopes of finding life elsewhere, find a fossilized living system as simple as possible is already a huge challenge.
| | 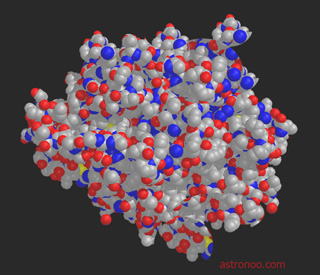 Image: Carbon is mass produced by nucleosynthesis (fusion of three helium nuclei) in the heart of massive stars, then released into space when they explode. Our chemistry, one that constitutes us, started with a small molecular edifice built on a carbon skeleton. When you see those collection ordered molecules, we understand the difficulty to recreate life in the laboratory.
Proteins are not living structures, and these already macromolecules, based on carbon, are extremely complex, they are formed by amino acids linked together by chemical bonds. The assembly of these molecules and their conformation that is, the order and the form of the three-dimensional structure, are of major importance because they determine the very specific actions of the molecule, chemical functions that are part of a manufacturing plan. The human perspiration, ether or vanilla are formed of the same molecules (carbon, oxygen and hydrogen), which differentiates them is their conformation. |
If we consider the problem from the primordial building blocks of ordinary matter, we note that the original matter, quarks, nucleons, atoms are agitated with the temperature, the heat also translates directly agitation of the particles. At absolute zero (-273.15 °C), the material is in a state of minimum energy, entropy is null and this results in a "total particle immobility", although in quantum physics, particles always have a non-zero amount of movement according to the Heisenberg uncertainty principle.
As soon as the absolute zero is reached, the particles agitate, organize and assemble in pure carbon (i.e. a single chemical species, H, N, Fe...), then in simple body or molecular single body (H2, N2, Fe...) before mixing with the game of chance with other species to form electrically neutral simple molecules, atomic aggregates to the particular forms, which share electrons (covalent bonds).
Until then the activity of the material is relatively well understood and at this stage we can not regard the matter as alive it simply assembled with the electromagnetic force based migration in the middle. The matter has no purpose, intention or particular project, it can be easily manipulated by the environment and by man when manufactured nanoparticles.
Molecular aggregates under the effect of thermal agitation and the random atomic jumps from one position to an adjacent position, form a variety of stable chemical compounds. More there will atoms in the middle and the higher the chance movement of atoms and molecules will put in order. This is the "order from disorder" referred Erwin Schrödinger (1887 - 1961) in his 1944 essay.
Among these naturally stable molecules, there will be solid molecules to crystalline structures and molecules devoid of crystal structure, that is to say that does not repeat. From a small number of atoms there will be an almost infinite number of building possibilities.
Each encounter between two atoms will depend on their electronegativity, i.e. their mutual ability to create an electromagnetic link. Chemical compounds are molecules of several different chemical elements, linked together by chemical bonds. | | Occasionally the chemical compounds self-organize into covalent chemical bonds.
The connections between the atoms are of 3 types:
- Ionic bonds: these are bonds resulting from the loss of one or more valence electrons (electrons of the peripheral layer occurring in chemical bonds) by one atom, and the uptake of these electrons with another atom. This type of bond is generally between elements having a difference of very high electronegativity ≥ 1.7 on the Pauling scale, between fluorine = 0.7 and francium = 4).
- Covalent bonds: each bonded atoms are sharing one valence electron to create a pair of electrons shared between two entities. elements linked in this type of connection have a difference in electronegativity, zero or <1.7 on the Pauling scale.
- Coordinate covalent bonds: these are covalent bonds between two atoms in which both electrons shared in the bond come from the same atom.
There seems to be from certain chemical compounds built on a carbon skeleton, wrapped in giant molecular structures, the activity of matter changes in nature. The assembly and the shape of these structures determine the chemical functions that will make parts of a manufacturing plan. This production plan has any information that will help to rebuild the system.
It was from that moment that life starts and the system is considered alive.
Hermann von Helmholtz (1821 − 1894) "If all our efforts to cause the birth of organisms from inert matter failed, it seems that we do so entirely fair wondering if, after all, life has never had an origin, if it is not quite as old as the material and whether its germs, transported from one celestial body to another, would not have developed wherever they found a favorable ground? "...
NB: electronegativity of an element is a quantity that characterizes its ability to attract electrons during the formation of a chemical bond with another element. | | 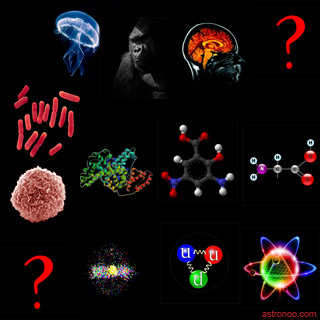 Image: synthesis of the long road of inert to living or the first particles to consciousness. The material combines in agglomerate, self-organizing chemically and complex constantly at the whim of electromagnetic links. It seems that life is a special form of the material both unpredictable and indefinable. But its tenacity is it not proof that it is present everywhere in the Universe, patiently waiting a favorable environment to continue its path to complexity?
There really is no free border between inert and living, the matter uses the principle of least action to build the living. The image reads between the two question marks, winding from the lower left to the upper right. The question mark at the bottom left is the big bang followed quarks, nucleons, atoms, molecules with amino acids and proteins, albumin, cell, archaea and bacteria, microorganisms, mammals, consciousness and the future. |



 Automatic translation
Automatic translation

 Artificial intelligence: the explosion of gigantism
Artificial intelligence: the explosion of gigantism
 When AI models train on their own data, they go mad!
When AI models train on their own data, they go mad!
 Emergence of artificial intelligence: Illusion of intelligence or intelligence?
Emergence of artificial intelligence: Illusion of intelligence or intelligence?
 The horseshoe crab, a living fossil!
The horseshoe crab, a living fossil!
 Biosignatures or presence of life in the Universe
Biosignatures or presence of life in the Universe
 Challenge and threat of Artificial Intelligence
Challenge and threat of Artificial Intelligence
 How do machines understand, interpret and generate language in a similar way to humans?
How do machines understand, interpret and generate language in a similar way to humans?
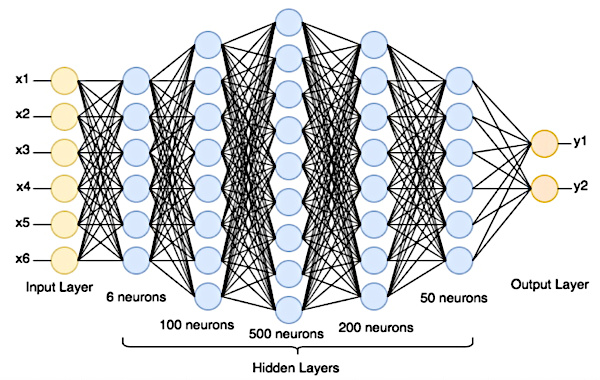 How does an artificial neural network work?
How does an artificial neural network work?
 Origin of life on Earth: Panspermia theory
Origin of life on Earth: Panspermia theory
 Origin of life on Earth: White smoker theory
Origin of life on Earth: White smoker theory
 Why 37 degrees Celsius?
Why 37 degrees Celsius?
 Thermodynamics of the sandpile
Thermodynamics of the sandpile
 Are we alone in the universe?
Are we alone in the universe?
 Trace of frozen life in Siberia
Trace of frozen life in Siberia
 Ice cores tell us about our past
Ice cores tell us about our past
 Life evolves in the shelter of glaciations
Life evolves in the shelter of glaciations
 Organ regeneration, the salamander
Organ regeneration, the salamander
 Cosmic rays and the mutation of species
Cosmic rays and the mutation of species
 Mephisto, the little worm of the depths
Mephisto, the little worm of the depths
 Discovery of solid buckyballs in space
Discovery of solid buckyballs in space
 Bipedalism in hominids
Bipedalism in hominids
 Kamchatka giant crab
Kamchatka giant crab
 The passage between the inert and the living
The passage between the inert and the living
 From particles to biochemical life
From particles to biochemical life
 Egocentric vision, the man at the center
Egocentric vision, the man at the center
 Megapod uses volcanic heat
Megapod uses volcanic heat
 Ardi is 4.4 million years old
Ardi is 4.4 million years old
 Natural selection, the birch moth
Natural selection, the birch moth
 The explosion of life in the Ordovician
The explosion of life in the Ordovician
 Liquid water, an accelerator of chemical reactions
Liquid water, an accelerator of chemical reactions
 Neandertal
Neandertal
 Asimo the future humanoid
Asimo the future humanoid
 Conditions for the appearance of life
Conditions for the appearance of life
 Fermi's paradox or Plato's cave
Fermi's paradox or Plato's cave
 The Tardigrade, the immortal animal
The Tardigrade, the immortal animal
 Toumaï, 7 million years old
Toumaï, 7 million years old
 Border between inanimate and living
Border between inanimate and living
 The incredible life of the abyss
The incredible life of the abyss
 Cyanobacteria create toxic gas
Cyanobacteria create toxic gas
 The short history of the evolution of life
The short history of the evolution of life
 The smallest frog in the world
The smallest frog in the world
 The explanation of the Little Ice Age
The explanation of the Little Ice Age
 Ashen light, the proofs of life
Ashen light, the proofs of life
 Bioluminescence of living organisms
Bioluminescence of living organisms
 Beyond our senses, the great scientific revolutions
Beyond our senses, the great scientific revolutions
 The primitive soup
The primitive soup