Radioactivity is a natural phenomenon ionizing present everywhere, which occurs in the nucleus, in the depths of the atoms. Unstable radioactive elements of the earth radiate much energy per disintegration.
Disintegration is the transformation of matter which naturally ejects particles and produces radioactive neutron radiation and energy. Uranium, thorium and potassium are the main radioactive elements responsible for the heat of the Earth. Although terrestrial radioactivity steadily decreased because radioactive atoms become stable elements, it remain still very important because uranium 238, thorium-232 and potassium 40 are long-lived elements (several billion years). Other radioactive elements those which have a very short period relative to the age of the Earth disappeared. And since its formation there is 4.2 billion years, the Earth gradually loses its intrinsic heat and cools down.
Earth drains continuously, a total power of ≈ 44 terawatts equivalent of 44,000 nuclear reactors. What is remarkable about observing our solar system, is the extraordinary diversity of objects connected to it. Yet they are all formed from the same cloud at the same place in the universe, from the same materials at the same time there are about 5 billion years.
How objects have been able to evolve so differently from identical initial conditions?
Astrophysicists have long sought to understand this phenomenon. And the most amazing is that an object has been able to create the conditions for the emergence of life and to have them preserved until today. If Astrophysics explains quite well the evolution of stars, it is far to explain the evolution of planets.
What are the conditions that led to the Earth so much complexity?
Is this the most complex object in the universe?
How planets absorbed energies so different? | | From the same initial state, all objects over time will evolve differently, they will gradually acquire an internal energy and will slowly lose it, depending of their mass. Indeed, the size of the object has a major importance in the accumulation of internal energy, astronomical objects are like energy tanks which empty gradually as the radioactive decay of its elements. The main active planets engine is the internal radioactivity which is converted into heat which rises from the center to the surface and maintains a certain activity (magnetism, volcanism, continental drift, recycle the atmosphere,...) on the planet.
The enormous solar energy is not sufficient to maintain our planet active because this energy is blocked at the earth's surface and does not penetrate the center of the Earth. The energy which do that the Earth is alive is trapped inside the planet, it is that of the radioactivity of uranium, thorium and potassium. If there was no radioactive decay, the Earth would be a dead planet. In summary, more the tank is bigger and more it will store energy. When the object is large it cools slowly. Thus small asteroids and comets have frozen there 5 billion years, the large asteroids have frozen there 4 billion years, the Moon has frozen there 3 billion years, Mars has frozen there to 1 billion years, the Earth after 4.2 billion years ago is still an active planet. More solar system objects are small (asteroids, comets) and more they interest to scientists because they spread all their energy into space and kept intact the materials at the time of their "dead", in particular organic molecules.
NB: natural radioisotopes: Americium 241, antimony 125, carbon-14, cesium-134, 135 and 137, chlorine-36, cobalt-60, curium 242 and 244, iodine-129, 131 and 133, krypton-85 and 89, phosphorus-32, plutonium-239 and 241, polonium-210, potassium-40, radium 226 and 242, ruthenium-106, selenium-75, sulfur-35, strontium-90, thorium-234, tritium 3, uranium 235 and 238. | | 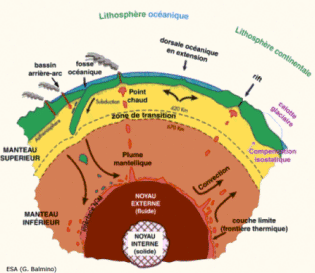 Image: The Earth is a huge reservoir of energy. This energy depths up to the surface by convection. The heat released by the natural radioactivity of deep rocks as uranium, thorium and potassium maintains a mild temperature on the surface since 4 billion years. The different layers of the Earth's are crust solid on the surface which has a thickness of 30-65 km, the upper mantle viscosity of 670 km, the lower elastic mantle of 2180 km, the liquid outer core 2270 km and the solid inner core 1220 km. |



 Automatic translation
Automatic translation
 Feynman diagrams and particle physics
Feynman diagrams and particle physics
 Stars cannot create elements heavier than iron because of the nuclear instability barrier
Stars cannot create elements heavier than iron because of the nuclear instability barrier
 What is β radioactivity?
What is β radioactivity?
 Planck wall theory
Planck wall theory
 Is emptiness really empty?
Is emptiness really empty?
 The Large Hadron Collider
The Large Hadron Collider
 The hadron is not a fixed object
The hadron is not a fixed object
 Radioactivity, natural and artificial
Radioactivity, natural and artificial
 The scale of nanoparticles
The scale of nanoparticles
 Schrodinger's Cat
Schrodinger's Cat
 Before the big bang the multiverse
Before the big bang the multiverse
 Eternal inflation
Eternal inflation
 Gravitational waves
Gravitational waves
 Principle of absorption and emission of a photon
Principle of absorption and emission of a photon
 Beyond our senses
Beyond our senses
 What is a wave?
What is a wave?
 The fields of reality: what is a field?
The fields of reality: what is a field?
 Space in time
Space in time
 Quantum computers
Quantum computers
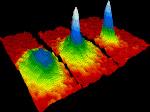 Bose-Einstein condensate
Bose-Einstein condensate
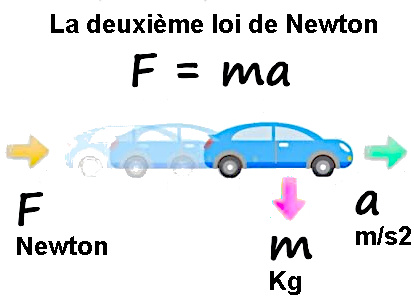 Equation of Newton's three laws
Equation of Newton's three laws
 Field concept in physics
Field concept in physics
 The electron, a kind of electrical point
The electron, a kind of electrical point
 Entropy and disorder
Entropy and disorder
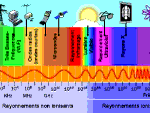 Light, all the light of the spectrum
Light, all the light of the spectrum
 The infernal journey of the photon
The infernal journey of the photon
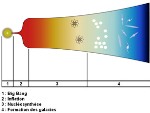 Mystery of the Big Bang, the problem of the horizon
Mystery of the Big Bang, the problem of the horizon
 The neutrino and beta radioactivity
The neutrino and beta radioactivity
 Einstein's space time
Einstein's space time
 The incredible precision of the second
The incredible precision of the second
 Why does physics have constants?
Why does physics have constants?
 Spectroscopy, an inexhaustible source of information
Spectroscopy, an inexhaustible source of information
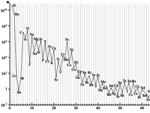 Abundance of chemical elements in the universe
Abundance of chemical elements in the universe
 Effects of light aberration
Effects of light aberration
 The size of atoms
The size of atoms
 The magnetic order and magnetization
The magnetic order and magnetization
 The quark confinement
The quark confinement
 Superpositions of quantum states
Superpositions of quantum states
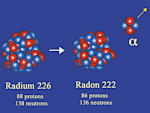 Alpha decay (α)
Alpha decay (α)
 Electromagnetic induction equation
Electromagnetic induction equation
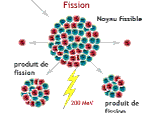 Nuclear fusion, natural energy source
Nuclear fusion, natural energy source
 Does dark matter exist?
Does dark matter exist?
 Non-baryonic matter
Non-baryonic matter
 The mystery of the structure of the atom
The mystery of the structure of the atom
 The mystery of matter, where mass comes from
The mystery of matter, where mass comes from
 Nuclear energy and uranium
Nuclear energy and uranium
 The Universe of X-rays
The Universe of X-rays
 How many photons to heat a coffee?
How many photons to heat a coffee?
 Image of gold atom, scanning tunneling microscope
Image of gold atom, scanning tunneling microscope
 Quantum tunneling of quantum mechanics
Quantum tunneling of quantum mechanics
 Entropy and its effects, the passage of time
Entropy and its effects, the passage of time
 The 12 particles of matter
The 12 particles of matter
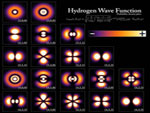 The atomic orbital or image atom
The atomic orbital or image atom
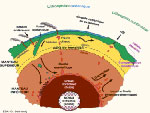 Earth's radioactivity
Earth's radioactivity
 The Leap Second
The Leap Second
 The vacuum has considerable energy
The vacuum has considerable energy
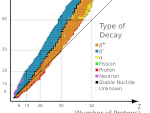 The valley of stability of atomic nuclei
The valley of stability of atomic nuclei
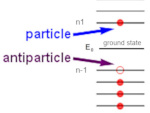 Antimatter and antiparticle
Antimatter and antiparticle
 What is an electric charge?
What is an electric charge?
 Our matter is not quantum!
Our matter is not quantum!
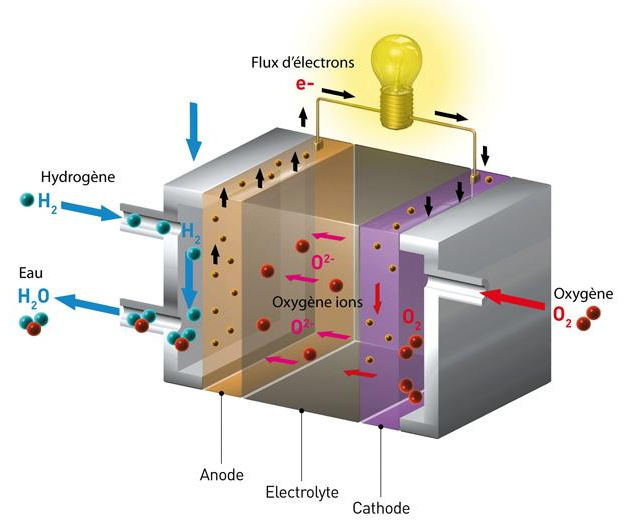 Why use hydrogen in the fuel cell?
Why use hydrogen in the fuel cell?
 The secrets of gravity
The secrets of gravity
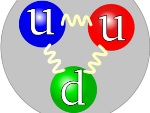 E=mc2 explains the mass of the proton
E=mc2 explains the mass of the proton
 Image of gravity since Albert Einstein
Image of gravity since Albert Einstein
 Einstein's miraculous year: 1905
Einstein's miraculous year: 1905
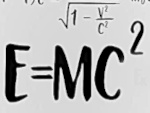 What does the equation E=mc2 really mean?
What does the equation E=mc2 really mean?
 Special relativity and space and time
Special relativity and space and time